Bovilis® BVD
The only BVD vaccine with a 12 month fetal protection claim*

Bovilis vaccines help farmers protect cattle from production losses and disease, giving them greater control of their herd’s health now and in the future.


The only BVD vaccine with a 12 month fetal protection claim*
The only commercially available vaccine against Moraxella bovis.
The only leptospiral vaccine developed, researched and trialled under New Zealand conditions for New Zealand farmers.

The one shot scours vaccine for all classes of stock
New Zealand’s only Salmonella vaccine for sheep and cattle.
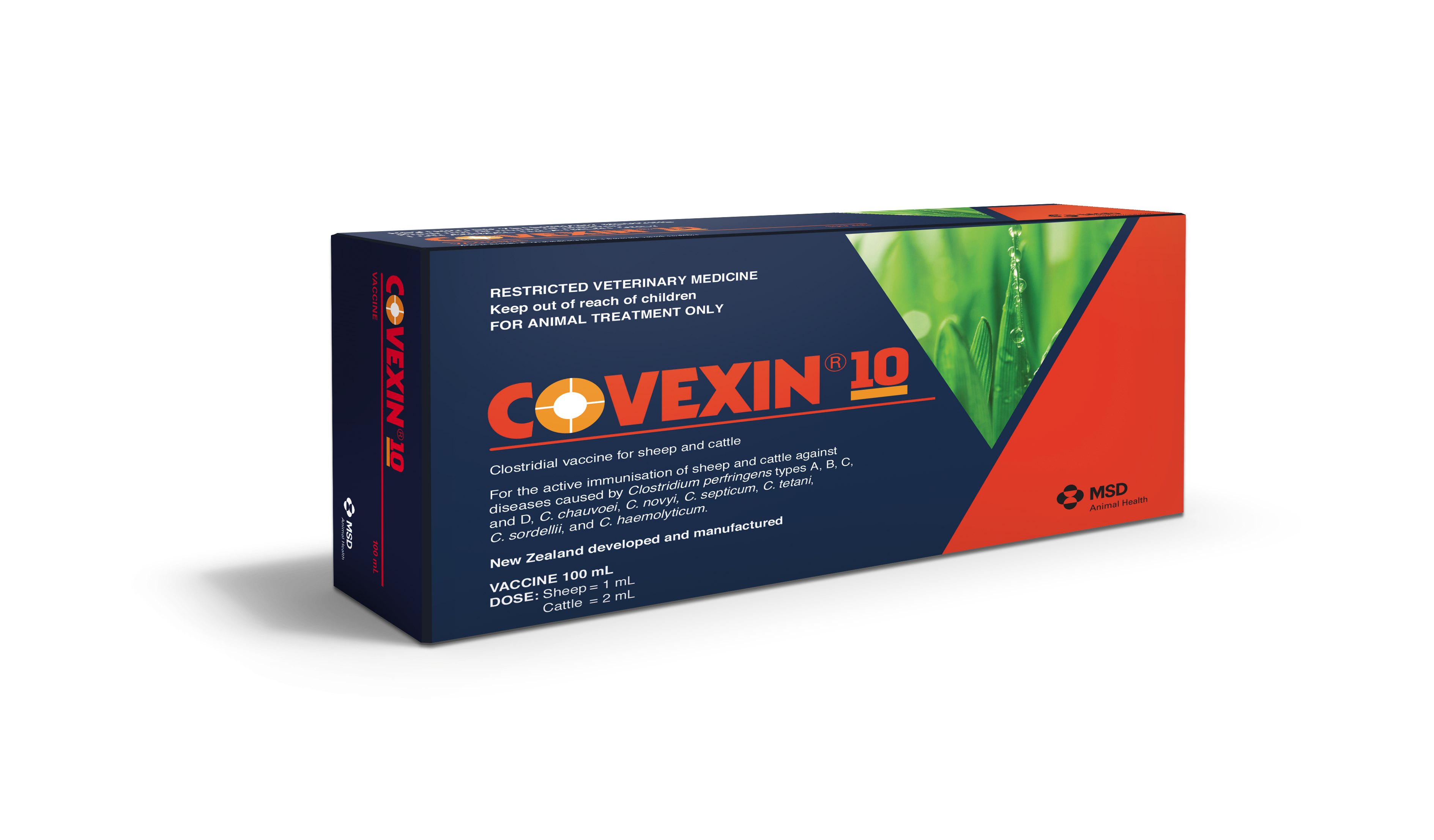
A 10-in-1 clostridial disease vaccine specially designed for high performance farms.

Vaccination of cows and heifers to aid in the reduction of clinical signs of cryptosporidiosis in calves.
No items to show.
BVD (Bovine Viral Diarrhoea) is a viral disease of cattle which is wide-spread in New Zealand. Affecting both dairy and beef herds, it is estimated that about half of herds are “actively-infected” with BVD at any given time1 and the cost of BVD to the New Zealand cattle industry is around $150 million a year.2
Symptoms of BVD include pregnancy loss, diarrhoea, milk drop, and reduced growth rates. It also suppresses the immune system, making animals more susceptible to other diseases, such as pneumonia and salmonella.
Research in New Zealand and overseas shows that it pays to control BVD; it is always more cost-effective to do something than to do nothing3. Moreover, a well-executed BVD control plan will help you achieve other farm goals, like improving animal welfare, reducing antibiotic usage, and improving reproductive performance.
How is BVD contracted?
BVD is spread by “persistently-infected” or “PI” cattle. The key to BVD control is therefore to find and eliminate PIs from within your herd, then protect your herd from contact with outside PIs. This can be accomplished by: monitoring the herd, testing individual animals, improving biosecurity, and strategically vaccinating ‘at-risk’ cattle.
Learn more about BVD, including how to test for and control BVD
1 Han, JH et al. (2018). Using Bayesian network modelling to untangle farm management risk factors for bovine viral diarrhoea virus infection. Preventive Veterinary Medicine. 161:75-82.
2 The BVD Management Toolkit. BVD Steering Committee
3 Weir, A. (2016). Epidemiology of BVD in New Zealand dairy herds. Massey University PhD thesis dissertation
Learn more about prevention with Bovilis® BVD vaccine
Neonatal diarrhoea is arguably one of the most common diseases of newborn calves worldwide. The consequences on animal welfare and those managing the calves can be devastating. The cost of mortality, poor growth and performance as well as treatment costs, all need to be considered. The infectious agents that cause neonatal calf diarrhoea are prevalent in New Zealand. In a recent survey 96% of farms in New Zealand had pathogens that can cause calf scours detected1.
For more information about calf scours and calf health in general click here
For information about prevention with Rotavec® Corona vaccine click here
